Falsified Medicines Directive
A European Union Directive introduced to safeguard patients and prevent falsified medicines from entering the legal supply chain.
Overview
The Falsified Medicines Directive (FMD) is a European Union Directive (2011/62/EU) aimed at preventing the distribution of falsified medicines. Since February 2019, safety features have been required on prescription medicine packaging to enable authentication of genuine products before they are supplied to patients. These ‘safety features’ include:
- Anti-tamper device: This ensures that the medicine packaging hasn’t been tampered with. While such devices were already common on many medicines, they are now mandatory for most medicines.
- Unique identifiers (2D barcode). The 2D barcode contains essential information about the medicine, including the product code, serial number, batch number and expiry date.
Tamper proof seal and 2D barcode
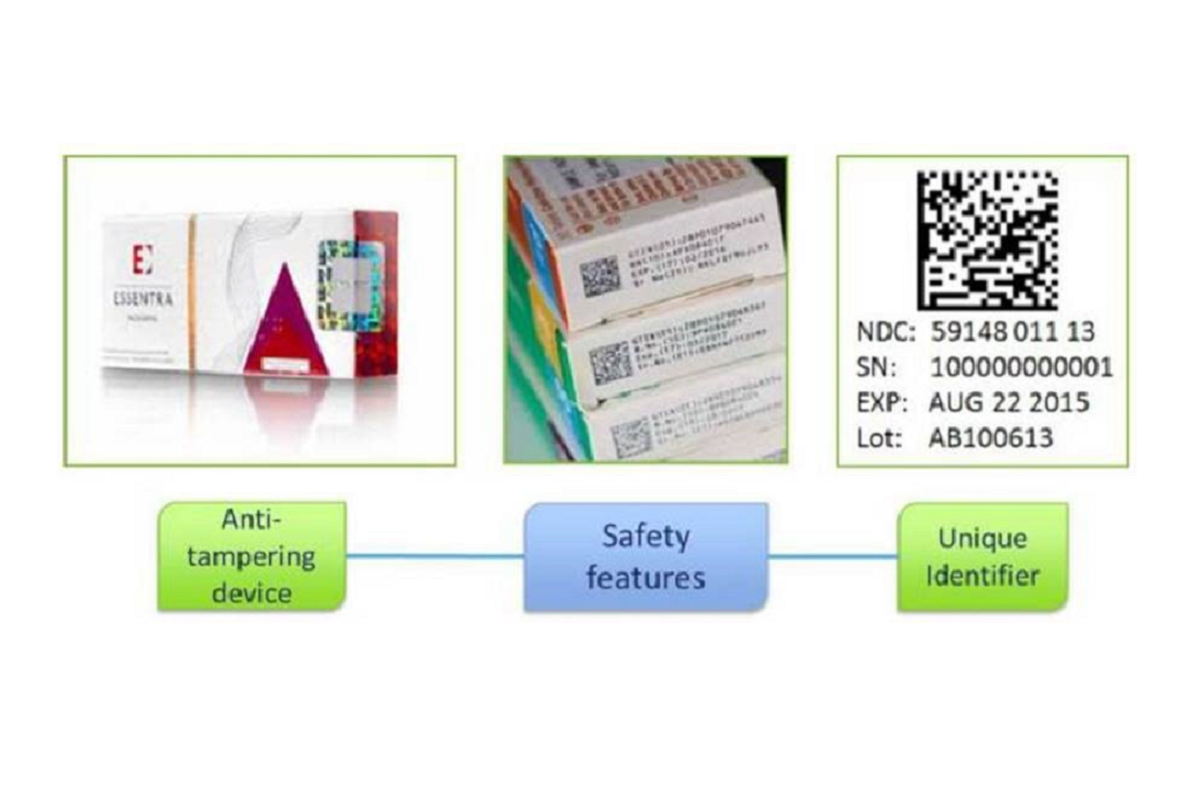
Responsibilities of pharmacists and pharmacies related to FMD
Pharmacists and pharmacies play an important role in ensuring compliance with FMD requirements. Here’s what they must do:
Verification and authentication
Before supplying medicines to patients' pharmacists must verify the safety features on the packaging of medicines:
- Authenticate the unique identifier (contained in the 2D barcode) by scanning the product and decommissioning it from the Irish Medicines Verification Organisation (IMVO) repository; and
- Check the anti-tamper device to ensure that it is intact.
To be able to scan and decommission the 2D barcode on a pack, pharmacies must have a scanner with associated software in place and be registered with the Irish Medicines Verification Organisation (IMVO), who was established to manage the medicines verification system for Ireland and connected to the Irish Medicines Verification System (IMVS).
Alert management
When scanning medicine packs, if an alert is generated (indicating a potential issue), the alert must be fully investigated, and a root cause identified, and falsification ruled out. It is important that alerts are closed out in a timely manner. The IMVO provides support to assist with Alert Management .
PSI compliance monitoring
The PSI monitors pharmacy compliance with FMD legislation (Commission Delegated Regulation on Safety Features (EU) 2016/161 by pharmacies) We carry out FMD inspections and engage directly with pharmacies and pharmacists where there is a concern as to their compliance.
Ongoing support
It is important for those in pharmacy governance roles, including all pharmacists, to stay informed about FMD requirements and actively participate in ensuring the safety and authenticity of medicines for patients.
Learning more about the Windsor Framework and FMD
The Windsor Framework, a post-Brexit legal agreement between the EU and the UK, will have an impact on several areas throughout Europe, including medicines. The Windsor Framework is set to take effect from 1 January 2025.
This framework will introduce changes affecting the FMD throughout Europe, particularly in countries like Ireland with close connections to the UK.
What will change in the UK due to the Windsor Framework?
FMD will no longer apply in Northern Ireland from 1 January 2025, having already been disapplied in the rest of the UK at the time of Brexit. The UKNI medicines verification system will cease to operate and the data in it will be deleted. Pharmacies, hospitals and wholesalers in Northern Ireland will be disconnected from the EU FMD system and will no longer have to scan packs.
For more details, visit the Irish Medicines Verification Organisation (IMVO) website. As more information becomes available, it’s important for you and your pharmacy team to stay informed.
The following guidance has been agreed between the Irish Medicines Verification Organisation (IMVO), Department of Health, Health Products Regulatory Authority (HPRA) and the PSI:
- It is expected that most UK packs supplied as exempt medicinal products (EMP) (also known as unlicensed medicines (or ULMs) in Ireland will continue to carry 2D barcodes and the only way to avoid an alert with these packs is not to scan them.
- If you inadvertently do scan a UK pack, you will get an amber or red alert message on your FMD software. Despite this, you may supply the pack unless:
- You have overriding concerns that a falsified medicine is involved or believe the pack has been interfered with; or
- The pack has expired. Your FMD software may not be able to flag that the pack is expired because of the UK system having been disconnected.
- Always check the anti-tampering device on the pack (if there is one). If you have any reason to believe the pack has been interfered with, please report this to the HPRA as a product quality defect and do not supply the pack. Email qualitydefects@hpra.ie to report this.
The Irish Medicines Verification Organisation (IMVO) is running a series of webinars for pharmacies, hospitals and wholesalers from now until early December 2024. These sessions will cover the Windsor Framework and how to prepare for it. It is recommended that you or a member of your team attend one of these webinars. Recordings and slides will also be available on the IMVO website.
Detailed guidance and ongoing support are available to pharmacies from the Irish Medicines Verification Organisation (IMVO).
- Queries on alerts: alert.support@imvo.ie
- Queries on end-user registration/connection to national system: registration@imvo.ie
- Tel: +353 1 5715320
Recorded webinars hosted by the Irish Institute of Pharmacy and IMVO can also provide helpful information.
- Health Products Regulatory Authority (HPRA)
- The HPRA website has information and updates about the FMD, relevant to its stakeholders.
- Queries: compliance@hpra.ie
- Telephone: +353 1 6764971
If a pharmacist or wholesaler has reason to believe that packaging has been interfered with, based on their examination of the anti-tampering device on the pack, they must report their concern to the HPRA and not supply the pack. The HPRA’s online reporting system (select the online form 'Medicine Quality Issue/Defect') should be used or email qualitydefects@hpra.ie.
For pharmacies in public hospitals and clinics, the HSE established a project team to co-ordinate the FMD implementation across the health services and will be managing communications within the HSE. For any FMD-related queries you can contact the project team: FMD.support@hse.ie
The IPU assists its members with FMD compliance and has published articles, reports and frequently asked questions providing more information on the FMD on its website (IPU Member access).
Falsified medicines are fake medicines designed to mimic real, authorised medicines. As falsified medicines do not pass through the usual evaluation of quality, safety, and efficacy required for EU authorisation, they can pose a real risk to patient safety.
Falsified medicines are different from counterfeit medicines. Counterfeit medicines are medicines that do not comply with intellectual property rights or that infringe trademark law.
The European Commission’s Delegated Regulation (EU) 2016/161, supplements the Falsified Medicines Directive 2011/62/EU, by setting out the safety features appearing on the packaging of medicines for human use. Most prescription medicines placed on the market since February 2019 are required to carry the following safety features:
- An anti-tamper device so the person supplying it can be sure it has not been interfered with.
- A unique identifier which is a 2D barcode containing information about the medicine. Embedded in this barcode will be a product code, serial number, batch number and expiry.
A pragmatic phased approach to the implementation of the legislation was taken with a 'use and learn' phase from February 2019 to May 2022.
The following prerequisites have been defined by the Safety Features Oversight Group* to ensure that end-users and marketing authorisation holders (MAHs) are ready for the end of use and learn, minimising disruption for them and for patients:
- Alert rate at 0.05% or lower, i.e., alerts generated by pharmacies, hospitals and wholesalers (‘end-users’), as a % of the total number of end-user scans**.
- All end-users scanning packs in accordance with their obligations under the Commission Delegated Regulation on Safety Features (EU) 2016/161 as amended.
- Avoidable alerts minimised by addressing issues with scanners, software, MAH data and procedural errors.
- Clear instructions available for all parties on what to do if there is an alert, including clear responsibilities for deciding if packs may be supplied or not, i.e., alert handling guidance in place.
- Fast efficient process for communication between parties.
- Clarity on impact of Brexit on supply chain/alerts; Capacity of end-users to deal with alerts in face of challenges of COVID-19.
- European Medicines Verification System has the capacity to cope with COVID-19 vaccines and treatments.
* Comprising Irish Medicines Verification Organisation (IMVO), Department of Health, Health Products Regulatory Authority (HPRA), Pharmaceutical Society of Ireland (PSI), Health Service Executive (HSE) and Private Hospitals Association (PHA).
**The following are excluded when calculating the % alert rate as they have no impact on Irish end-users - alerts generated by MAH transactions and alerts from intermarket transactions (IMTs), i.e. alerts on Irish packs generated by end-users in other markets.
Under the EU directive, hospitals are considered “Healthcare Institutions” and are required to verify the safety features on medicinal products. The Directive provides for healthcare institutions to scan and decommission products at an earlier point to assist with local logistical arrangements. The HSE is leading centrally on the introduction of the Falsified Medicines Directive in public hospitals. The HSE has a central contact point and ask anyone affected by FMD to send your questions / concerns to: FMD.support@hse.ie. The Private Hospitals Association has also been working with the Department of Health stakeholder group to prepare the private hospitals for introduction of FMD: info@privatehospitals.ie.
It is the responsibility of pharmacy owners and superintendent pharmacists to ensure that the legal requirements of FMD are being met within their pharmacies to ensure the continuity of safe supply of medicines to patients.
The Department of Health and the PSI are consulting on improving FMD compliance in pharmacies and are drafting legislation to invoke regulatory enforcement measures.
S.I. No. 270 of Medicinal products (safety features on packaging) Regulations 2022 was signed into legislation by the Minister of Health on 1 June 2022. This legislation makes verification and decommission of medicinal products a legal requirement.
The Delegated Regulations on Safety Features came into effect across Europe on 9 February 2019. Irish legislation giving effect to Commission Delegated Regulation (EU) 2016/161 commenced on 8 February 2019.
The Commission Delegated Regulation (EU) 2016/161 (‘Delegated Regulation’) was published by the Commission in February 2016. This legislation sets out detailed rules for the safety features appearing on the packaging of medicinal products for human use. It supplements the Directive and sets out exactly what manufacturers, wholesalers and persons authorised to supply medicines to the public need to do to ensure that the medicines supplied to patients are authentic.
The publication of the Delegated Regulation was accompanied by the publication of documents including a Q&A document from the EU Commission.
In 2011, the European Commission published a new directive, 2011/62/EU, known as the Falsified Medicines Directive which amends EU Directive 2001/83/EC on Human Medicinal Products. The purpose of this Directive is to introduce a coordinated and IT-enabled system of measures across Europe designed to protect the medicines supply chain against the risk of falsified (or counterfeit) medicines. These measures are intended to enhance patient safety by protecting the pharmaceutical supply chain from infiltration by falsified (or counterfeit) medicines and by introducing new rules to, more rigorously, regulate the supply chain.
The Falsified Medicines Directive (2011/62/EU) is a patient safety initiative that introduced new requirements to prevent falsified medicines from entering the medicines supply chain.
You can review PSI communications to pharmacists on FMD below:
- 5 February, 8 May, 2 September and 1 October 2019
- 7 February 2020
- 12 November 2021
- 23 December 2021
- 27 May 2022 - read the PSI email noting the end of 'use and learn' phase on 30 May 2022
- 21 December 2022

