Open Disclosure
Open disclosure is about taking an open and honest approach to communicating with patients and their families when things go wrong in healthcare
New patient safety legislation has been commenced that requires mandatory open disclosure processes in specific, rare, but very serious, circumstances. Additionally, a new national open disclosure framework has been produced that sets out requirements and expectations across all health and social care settings and seeks to ensure a clear and consistent approach to open disclosure in practice.
The PSI is pleased to share a training programme specifically on open disclosure in pharmacy is now available through the IIOP.
What is open disclosure
Open disclosure is an open, honest, empathic, and timely approach to communicating with patients and their families when things go wrong in healthcare. This includes expressing regret for what has happened, keeping the patient informed, and providing reassurance in relation to on-going care and treatment, learning, and the steps being taken by the provider to prevent a recurrence of the incident.
Sometimes, and in other jurisdictions, you might see open disclosure referred to as ‘open communication’, ‘duty of candour’, or ‘being open’.
The principles of open disclosure are captured in the PSI’s Core Competency Framework and in the Code of Conduct, where open and honest communication, legal and ethical practice, and person-centred care are incorporated throughout.
What are the legislative provisions around ‘Open Disclosure’?
The Patient Safety Act
On 26 September 2024, the Patient Safety (Notifiable Incidents and Open Disclosure) Act 2023 commenced. This Act provides a legislative framework for mandatory open disclosure and aims to embed openness and transparency in healthcare settings in Ireland, including in private healthcare. The Act contains a list of very rare, very serious incidents. These specific notifiable incidents are explicitly bound by legislation in how they are responded to and are subject to mandatory reporting through the National Incident Management System (NIMS). You can find more information on NIMS on the HIQA website, including specific guidance for health service providers on how to notify HIQA of a notifiable incident under the Patient Safety Act.
The Department of Health has also developed a guidance document to assist stakeholders in understanding the provisions of the Act.
The National Open Disclosure Framework – The ‘Framework’
In addition to the legislative requirements for defined notifiable incidents, the National Patient Safety Office (NPSO) of the Department of Health developed The National Open Disclosure Framework.
The Framework seeks to further embed structures for transparency and open communication from all health and social care professionals with patients following a patient safety incident or an adverse event.
The Framework provides six overarching principles that are expected to be used to underpin open disclosure in practice, including in pharmacy practice. You can read more about these in the Framework.
What do the developments in open disclosure mean for a pharmacist or a pharmacy owner?
A patient-facing Pharmacist
All patient-facing pharmacists must ensure they are aware of the new legislation, the framework, and the overall expectations should a patient safety incident or adverse event arise.
A Pharmacy Owner and Superintendent and/or Supervising Pharmacist
Pharmacists in governance roles and pharmacy owners are required to ensure robust policies are in place which underpin open disclosure practices and support a culture of open disclosure in the pharmacy. Pharmacists and relevant members of the pharmacy team must receive training on the open disclosure policies that are in place and on how to appropriately respond to patient safety incidents, such as medication errors, in the pharmacy.
Policies and Procedures in your Pharmacy
The Framework provides guidance on open disclosure policy requirements for pharmacies as health and social care providers.
At a summary level, the Framework requires that open disclosure policies in the pharmacy should ensure that the pharmacy team, as relevant to their roles, understand:
- what open disclosure means,
- what types of incidents require open disclosure,
- the information that must be provided to patients,
- how open disclosure processes should be managed,
- what is disclosed and how it works
The policies should:
- empower the pharmacy team to report patient safety incidents and adverse events
- set out how to communicate with patients and their support persons openly in relation to any such incidents.
- ensure that open disclosure is managed in a manner that is compassionate, caring and empathetic toward all those involved in and/or affected by patient safety incidents or adverse events.
Levels of Response
The Framework includes an open disclosure process diagram (see below) which outlines all possible considerations when designing an open disclosure process and pharmacies should update or design their policies with this in mind.
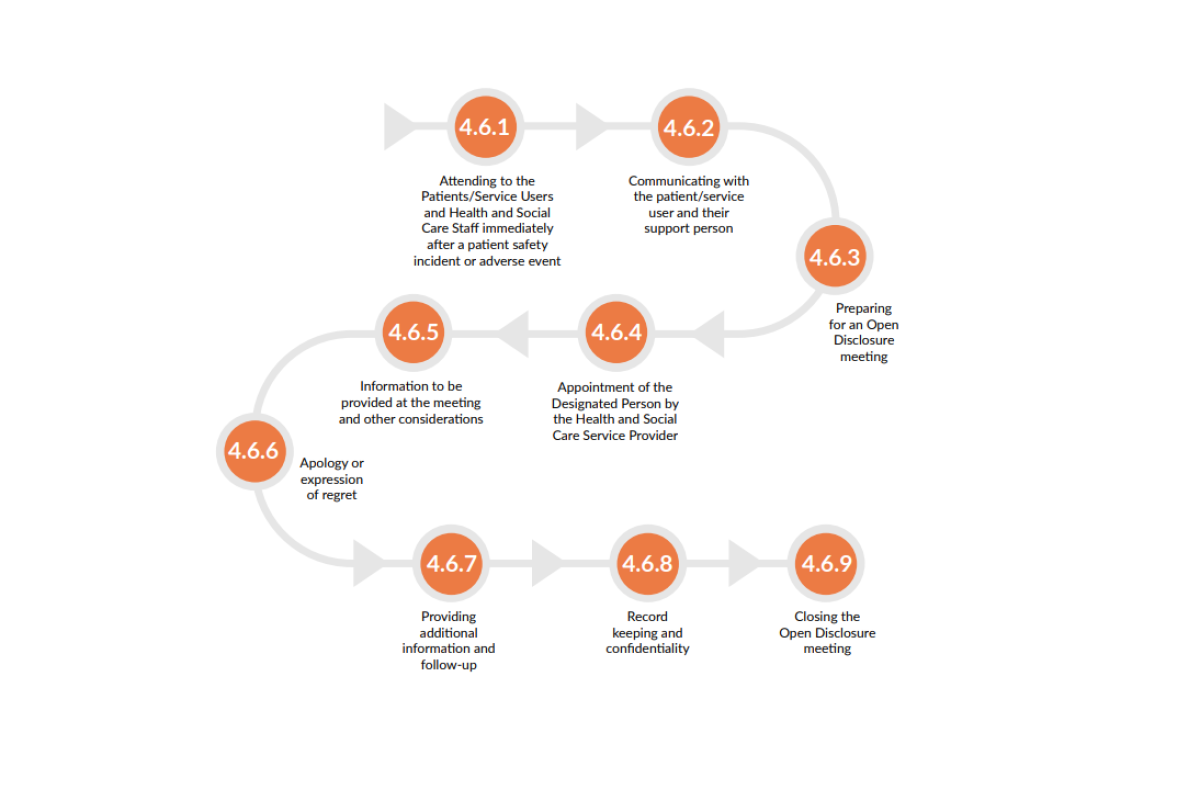

Guidance is provided on each of the steps in the Framework. The Framework notes that different approaches may be taken to open disclosure, depending on whether a ‘low’ or ‘high’ level response is required. The level of response required will be defined by the degree of harm the patient has experienced, the level of additional interventions or treatments required because of this harm, and the expectations of the patient and their support person. Responses may vary from one open disclosure meeting to a number of meetings, for example.
For those incidents requiring a high-level response, pharmacists must first determine whether the incident is a ‘notifiable incident’, as listed under Schedule 1 of the Patient Safety (Notifiable Incidents and Open Disclosure) Act 2003. Notifiable incidents must be responded to as set out under the Act.
Additional considerations for policy development, as set out in the Framework, include but are not limited to:
- Provision of appropriate medical care, treatment, and support services to patients following patient safety incidents.
- Requirements revolve around timely communication with the patient and their support person.
- Communicating with younger people under the age required for medical consent and/or other vulnerable persons.
- The role of a designated person for dealing with clarification requests received from the patient and to maintain a seamless line of communication between the patient, the support person, and the pharmacy.
- Preparation for and provision of information at open disclosure meetings.
- Expectations around apologies and expressions of regret.
- Record-keeping and confidentiality requirements.
- Processes for the provision of additional information, on-going communication and follow-up care.
- How to close out an open disclosure process, including consideration of whether a summary of the process should be provided to the patient and their support persons, asking if there are any outstanding concerns or complaints about the process, and consideration of any further action arising from this.
- Support requirements for the pharmacy team following patient safety incidents.
A culture of open disclosure
The superintendent pharmacist and pharmacy owner, in partnership with the supervising pharmacist, are tasked with establishing and embedding a culture of openness in the pharmacy. The Framework notes that successful implementation of a culture of open disclosure will require:
- Policies/systems for monitoring and continuous learning and improvement around open disclosure.
- Leadership and designation of an ‘open disclosure champion’ who can lead and promote open disclosure policy, education/training, and monitor practice.
- Opportunities for open communication, engagement, and feedback from all pharmacy team members as well as patients/support persons, including mechanisms to assess how well the culture and practice of open disclosure are working.
- Training and development for all pharmacy team members.
The role of PSI
The Department of Health is responsible for overseeing the implementation of the National Open Disclosure Framework 2023 and as the pharmacy regulator, the PSI has obligations under the Framework. Amongst these requirements, it involves monitoring and reporting on the implementation of the Open Disclosure Framework, including that pharmacies have appointed an ‘open disclosure champion’ and have a documented policy or Standard Operating Procedure (SOP) around managing patient safety incidents, including an associated training and support structure for all pharmacy staff. The Framework provides clear guidance on requirements for these policies and the expectations which must be met.
In support of our obligations, a comprehensive training programme focused on Open Disclosure in pharmacies is on the IIOP’s e-learning platform. It aims to raise awareness amongst all pharmacists—particularly those in community pharmacy settings— about their responsibilities under the Framework.
We have already incorporated updates into the inspections undertaken for new pharmacy openings since mid-September 2025. Further integration into other inspection formats will commence from January 2026. This will include reviewing how pharmacists and pharmacies are meeting their obligations under the Framework. As the statutory body responsible for receiving complaints about pharmacists and pharmacies, in our report to the Minister for Health on our requirements, we must also include data on any complaints received that relate to open disclosure and, where appropriate, an analysis of these matters.
All relevant activities and data are included in our annual report to the Minister for Health.
You can find the inaugural stakeholder report from the Department of Health here.
Irish Institute of Pharmacy (IIOP)
A training programme on open disclosure is now available through the IIOP. It is aimed at those working in community pharmacy, to support a clearer understanding of responsibilities.
All patient-facing pharmacists must ensure they are aware of their obligations under the patient safety legislation that commenced in later 2024 and outlined in the accompanying National Open Disclosure Framework. Those in governance roles, including non-pharmacist pharmacy owners, are further tasked to ensure robust policies are in place which underpin open disclosure practices and support a culture of open disclosure in their pharmacy.
The training now available consists of two modules:
Module 1: Covers the principles of open disclosure and outlines professional, regulatory, legal, and ethical obligations.
Module 2: Focuses on the open disclosure process and documentation requirements following patient safety incidents, including those involving no harm, minor harm, moderate harm, and significant harm.
If you have any queries around the PSI’s role and expectations for open disclosure, please contact ProfessionalStandards@psi.ie
Additional supports can be accessed through the following:
For hospital pharmacies, there are also two e-Learning modules around Open Disclosure, which can be accessed free of charge on HSElanD.ie
- E-Learning Programme Module 1 – “Communicating Effectively through Open Disclosure”
- E-Learning Programme Module 2 – “Open Disclosure: Applying Principles to Practice”
HSE
The HSE has its own open disclosure policy and guidance documents, which correlate to the new national framework and the Patient Safety (Notifiable Incidents and Open Disclosure) Act 2023.
You can find more information on the HSE policy and guidance here.
The National Patient Safety Office (NPSO)
The NPSO facilitates monthly webinars on Open Disclosure related topics. You can attend the live webinars without registration, and they are also available to be watched back at your convenience.
You can find the list of upcoming and past webinars here.
The NPSO has also set up a dedicated support email to address all queries related to the new Open Disclosure Framework: opendisclosureframework@health.gov.ie

