Emergency Medicines Training
Training for Pharmacists for the Supply and Administration of Emergency Medicines
Pharmacists who have completed the required training, are permitted to administer five medicines for the purpose of saving life or reducing severe distress in certain emergency situations:
- anaphylaxis (adrenaline),
- asthma attack (salbutamol inhaler),
- hypoglycaemia (glucagon injection),
- angina attack (glyceryl trinitrate aerosol),
- opioid overdose (naloxone).
Read the legislation which allows for these services.
How can I complete training?
You can find more information about training and providers on the Irish Institute of Pharmacy (IIOP) website. There you will find details on how to register and complete most of the required training programmes. Many of the training programmes are available online as e-learning programmes. There is a fee associated with the Medicines Administration (Parenteral) (PAMT) training programme which is a blended programme, involving both online and face to face components.
What training do I need to complete?
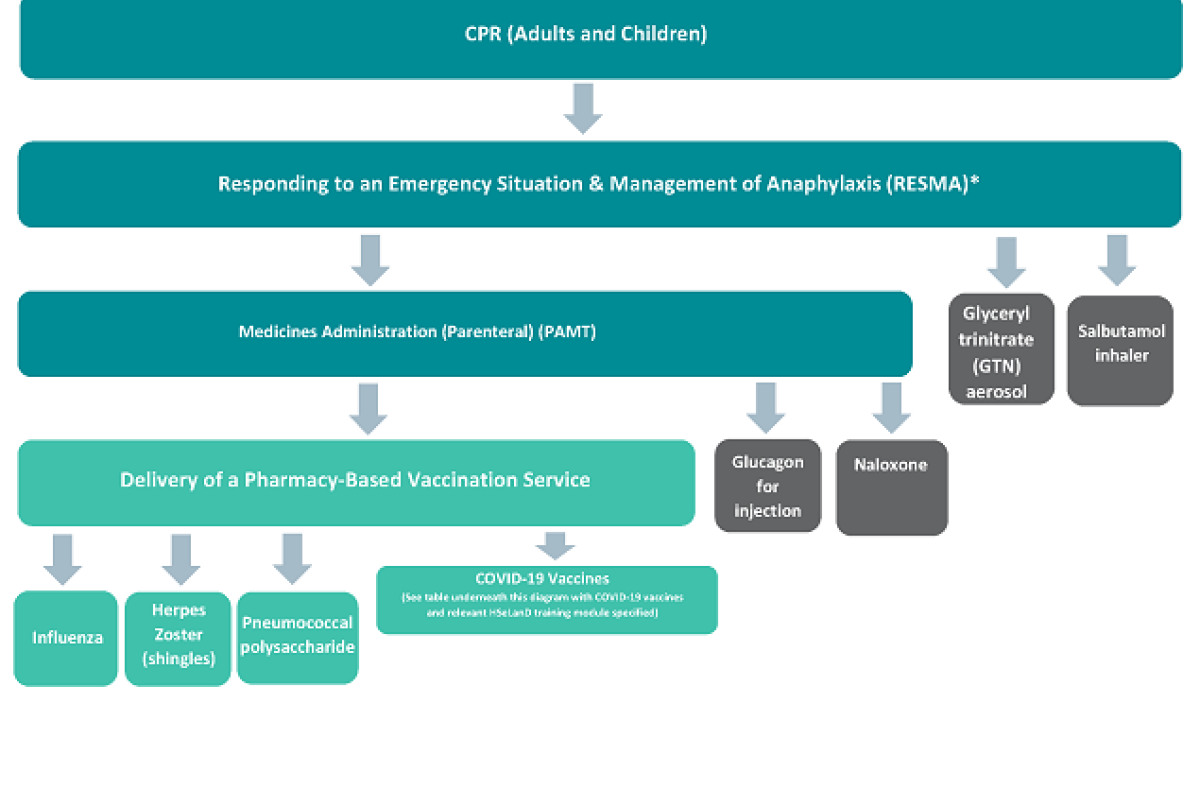
The training programmes you need to complete will depend on what services you wish to provide. A diagram of the training programmes is set out below. This is known as a ‘modular’ system of training. Some of the training programmes are the same as those required to supply and administer vaccinations. If you have competed training in these programmes previously and still have a valid certificate for this training, you do not have to complete the training again. Further information on vaccination services training requirements is available.
The training programmes for the specific emergency medicines you wish to administer can then be completed. This is illustrated in the diagram below.
Diagram illustrating pharmacist training requirements for the supply and administration of vaccines and emergency medicines
COVID-19 Vaccinations used in the COVID-19 Vaccination Programme and relevant HSeLanD module to be completed as part of the required training
| COVID-19 Vaccine |
HSeLanD Module |
|---|---|
| Comirnaty JN.1 3mcg (for children aged 6 months to 4 years) | mRNA COVID-19 Vaccine Formulations for children aged 6 months to 4 years |
| Comirnaty KP.2 3mcg (for children aged 6 months to 4 years) | mRNA COVID-19 Vaccine Formulations for children aged 6 months to 4 years |
| Comirnaty JN.1 10mcg (for children aged 5-11 years) | mRNA COVID-19 Vaccine Formulations for children aged 5-11 years |
| Comirnaty JN.1 30mcg (for individuals 12 years and older) | mRNA COVID-19 Vaccine Formulations for people aged 12 years and older |
| Comirnaty KP.2 30mcg (for individuals 12 years and older) | mRNA COVID-19 Vaccines Formulations for people aged 12 years and older |
| Nuvaxovid XBB.1.5 | COVID-19 Vaccination Training Programme – Nuvaxovid XBB.1.5 |
*The National Immunisation Advisory Committee (NIAC) made significant changes to the Anaphylaxis Chapter of the Immunisation Guidelines for Ireland in June 2022, which stated that “Adrenaline auto-injectors are not recommended as first line treatment by health professionals for the immediate management of anaphylaxis or suspected anaphylaxis following vaccination unless they are the only source of adrenaline available, as they may not allow IM delivery of an age appropriate dose”.
In the absence of any other National Guidelines for the immediate management of anaphylaxis in the community, PSI would consider it best practice for a pharmacist to administer Adrenaline (Epinephrine) intramuscularly from an ampoule, in all emergency circumstances (where indicated), in accordance with NIAC guidelines.
This would require the pharmacist to have valid training in:
- CPR,
- RESMA, and
- PAMT
However, if only an Adrenaline (Epinephrine) auto-injector is available, or if you are only trained and competent to administer an Adrenaline (Epinephrine) auto-injector, this should be used.
Pharmacists should use their expert knowledge, skills and professional judgement to administer Adrenaline (Epinephrine) in line with national guidance in accordance with the product readily available to them, which they are trained and competent to administer.
How can I access training and how long is it valid for?
| Training programme | Validity and where to access |
|---|---|
| CPR Course for adults and children | Details of providers are available on the IIOP website. Training is valid for two years (or as stated by training provider) |
| Medicines Administration (Parenteral) (PAMT) training programme | Available as a blended programme through Hibernian Healthcare. Pharmacists are asked to reflect, self-assess and to evaluate whether they need to refresh their training in this programme, in order to ensure they have the necessary skills and knowledge to safely deliver the associated medicines or vaccination service. The PSI requires that this training programme is repeated if a pharmacist wishes to administer: 1. a vaccine or emergency medicine via an injection route i.e., intramuscular or subcutaneous, which they have never previously administered; or 2. a vaccine or emergency medicine via an injection route which they have neither practised (i.e. administered to a patient) nor been trained on in the previous 12 months (or in the case of seasonal influenza vaccination, in the previous flu season) |
| Responding to an Emergency Situation and Management of Anaphylaxis (RESMA) training programme | Developed by Hibernian Healthcare and available through the IIOP. Training is valid for two years. |
| Delivery of a Pharmacy-based Vaccination Service Training Programme | Available through the IIOP. Pharmacists are asked to reflect, self-assess and to evaluate whether they need to refresh their training in this programme, in order to ensure they have the necessary skills and knowledge to safely deliver a vaccination service. The PSI requires that this training programme is repeated if a pharmacist has not vaccinated in the past 12 months (or influenza season). |
| Training for specific vaccines/emergency medicines to be administered | Available either through the IIOP (influenza, pneumococcal and herpes zoster (shingles) vaccines), or in the case of the COVID-19 vaccines and Naloxone through HSeLanD. Influenza training is valid for one year. Pneumococcal and Herpes Zoster training is valid for two years. |
| Naloxone Pharmacists are required to undertake the HSeLanD online module entitled 'Opioid Overdose Awareness and Naloxone Administration Training (Module 1)', in addition to maintaining up-to-date training in: - CPR - Responding to an Emergency Situation and Management of Anaphylaxis (RESMA) - Medicines Administration (Parenteral) (PAMT) This means that pharmacists can build on their existing skills they have developed through the delivery of vaccinations, and no longer have to undertake specific face-to-face training to supply and administer Naloxone in emergencies. Face-to-face training (Module 2) is also available through the HSE, should pharmacists wish to complete it, though this is not a mandatory requirement. |
Emergency Medicines
| Glyceryl Trinitrate Spray | Two years - Available through IIOP |
| Salbutamol inhaler | Two years - Available through IIOP |
| Glucagon | Two years - Available through IIOP |
| Naloxone |
Two years - Available through HSELand. Pharmacists are required to undertake Module 1 entitled ‘Opioid Overdose Awareness and Naloxone Administration Training’. |
What do I need to do each year?
You should review the training requirements for the delivery of the service(s) you wish to provide each year. You should check that your training in each training programme is up-to-date. You should also complete a self-assessment and self-declaration form, which will help you to review the requirements and provide a means for you to attest to your competency to deliver the chosen service(s).
If you have any questions about the training requirements, you can email cpd@psi.ie
In addition to completing the above training pathway, pharmacists should ensure that they are familiar with the most recent versions of both the NIAC and NIO national guidance documents on management of anaphylaxis. Pharmacists should be aware of updates to relevant national guidance and adapt their practices to reflect the most up to date information.
Review of Vaccination and Emergency Medicines Training Requirements for Pharmacists 2025
We have committed to reviewing the current vaccination and emergency medicines training requirements for pharmacists in our Service Plan 2025.
As part of this review, we are conducting research and engagement with pharmacists and a range of stakeholders. We will use this information to make recommendations on how the training requirements could be changed or improved to ensure they continue to be appropriate and proportionate.
Pharmacist Survey (April/May 2025)
During April and May 2025, we gathered feedback from pharmacists on the current vaccination and emergency medicines training requirements.
The feedback was gathered to help us determine if the current training requirements are appropriate in continuing to support pharmacists to deliver safe, high-quality vaccination and emergency medicines services. A report with the feedback from the survey, together with research and insights from stakeholder engagement is currently being drafted for consideration by the Regulatory and Professional Policy Committee and PSI Council. More information will be available in due course.

